Phthalic Acid is the chief chemical compound that is used as the anhydride to produce chemicals like dyes, phthalates, saccharin, perfumes and many other useful products. Phthalic Acid is an isomer of isophthalic acid and terephthalic acid. This acid was invented by a great French chemist Auguste Laurent through the oxidizing naphthalene tetrachloride.
Properties Of Phthalic Acid
| Chemical formula | C8H6O4 or C6H4(COOH)2 or H2C8H4O4 |
| Molecular weight | 166.132 g/mol |
| Density | 1.593 g/cm3 |
| Chemical names | ortho-Phthalic acid, 1,2-Benzenedioic acid,
Benzene-1,2-dioic acid |
| Solubility in water | 0.6 g / 100 mL |
| Melting point | 207 °C |
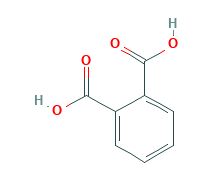
Phthalic Acid Structural Formula
Phthalic acid is a benzene dicarboxylic acid consisting of 2 carboxy groups at ortho positions. It has a role as a human xenobiotic metabolite. It is the conjugate acid of a phthalate(1-) and a phthalate. The chemical structure of phthalic acid is shown in the figure below.
For more information on any chemical compound, refer BYJU’S.
Comments